How to Find Your Way Around the Cell and Gene Therapy Space
Getting life saving C> products to patients is already complicated enough. Figuring out where different companies fit in the sector should be the easy part. If you provide services like contract research, contract manufacturing, clinical research then this page is designed help you organize different clients or prospects into mental boxes.
We hope you'll find the resources below to be helpful in your own learning about the sector.

Have a company in mind already?
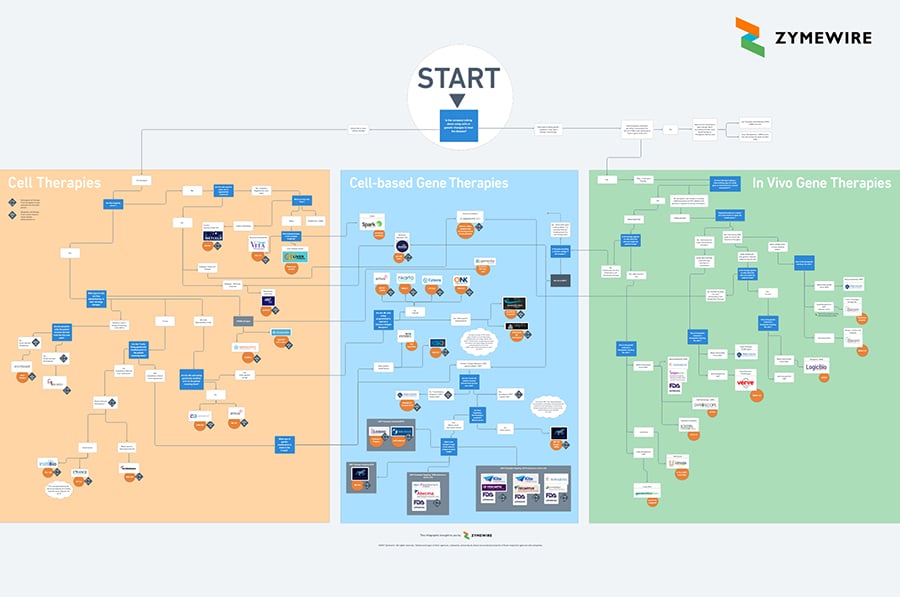
Start at the top of the map and take the company or product through the different questions. You should end up in the region of other peers and the most similar cell or gene therapies.

Just looking to browse the sector?
Use the Zoom button in the bottom right corner of the map to zoom into an area of interest. Check out the Scavenger Hunt below the map to try and find FDA approved cell therapies and other up and coming areas.

What is the difference between cell therapy and gene therapy? Learn from example programs
Each region of the map has one or more real world example programs.
How to zoom around
Scavenger Hunt
Try finding these specific points on the map as a way to learn more about the key technologies and companies

In vivo CAR-T. It's a thing.
Umoja is one to watch. You can find it down the Gene Therapy branch, but unlike all its CAR-T peers, Umoja is attempting CAR-T without involving any of the cell processing.
VivoVec UB-VV100 + RACR-CD19 CAR in vivo CAR-T targeting CD19
Find the approved CAR-T therapies.
You'll see that the approved CAR-Ts are all quite closely related, with two of them coming from the same company and targeting the same cell membrane protein (CD19).

Allogeneic CAR T is an emerging field to watch.
Look for Precision Bioscience on the map and see where it lives relative to more traditional CAR T programs.
PBCAR0191 Allogeneic CAR T therapy
The same gene therapy company can be pursuing multiple modes of delivery.
Look for Beam Therapeutics under the Gene Therapy section. Beam is increasing their chances of success by employing a lipid nanoparticle approach as well as an electroporation approach to effecting genetic change in target cells.

Find out! Request your PDF below.
Learn from expert relationship builders in the cell and gene therapy space.
In this video you'll hear about the key differences to think about when you're interacting with Cell and Gene companies compared to small molecule and recombinant protein drug developers.
Nick Stephens, President of The RSA Group, shares his observations on the patient-centric mission that Cell and Gene companies tend to have.
Janel Firestein from Clarkston Consulting shares her experiences around the higher data and digital needs that Cell Therapy companies tend to have, because of their need for transparency in the supply chain.
Sergio Armani, VP Business Development at Advarra, shares some of the challenges he sees from his vantage point related to the clinical complexity of running a gene therapy study. Sergio also shares guidance on IBC Reviews.





Questions to help organize cell and gene therapy (ATMP) companies
Is the company talking about using cells or genetic changes to treat the disease?
1. Using Cells or uses "cellular therapy". Are they targeting cancer?2.1. Yes. They are working on cancer. What type of cells are they administering in their oncology therapy?
2.1.1.. Dendritic cells. Are the dendritic cells the patient receives derived from her/his own cells?
2.1.1.1. No. They are donor-derived cells (allogeneic). An example here of allogeneic dendritic cells is Lineage Cell Therapeutics' VAC2 Product.
2.1.1.2. Yes. The cells are from the patient (autologous). An example here of a company working on autologous dendritic cells is APAC Biotech.
2.1.2. T-Cells. Are the T-cells being genetically modified prior to the patient receiving them?
2.1.2.1 No. Sometimes this is referred to as 'optimized' or 'non-engineered.' Are the T-cells being used to target solid tumors or blood cancers?
2.1.2.1.1 Solid Tumors. Examples here include InstilBio's ITIL-168 and Iovance's LN-144.
2.1.2.1.2 Blood cancers. An example here is NexImmune's NEXI-002, which the company is using to target Multiple Myeloma.
2.1.2.2. Yes, the cells are being genetically modified. This is sometimes referred to as "engineered.' What type of genetic modification is made to the T-cells?
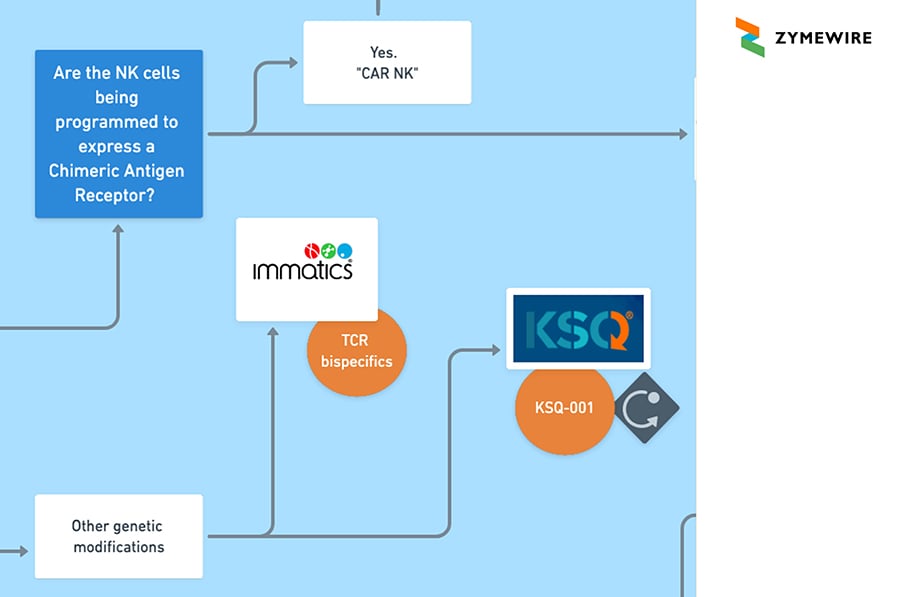
2.1.2.2.1 Other genetic modifications. Immatics/ TCR bispecifics. KSQ / KSQ-001
2.1.2.2.2 Chimeric Antigen Receptor (CAR) gene(s) added: CAR-T. Are the T-cells the patient receives from her/his own cells?
2.1.2.2.1.1 No. From Donors Allogeneic CAR-T (newer fields). Precision Biosciences / PBCARD191 (targets CD19)
2.1.2.2.1.2 Yes. Autologous CAR-T “Classic CAR”. Are they targeting a Hematological condition? (blood cancer)
2.1.2.2.1.2.1 Yes. Blood cancer. (aka liquid tumors). What is the (antigen) target of the chimeric antigen receptor (CAR)?
2.1.2.2.1.2.1.1 CAR-T Examples Targeting BCMA.
2.1.2.2.1.2.1.1.1 Legend Biotec / Cartitude-1 Program.
2.1.2.2.1.2.1.1.2 Arcellx / CART-ddBCMA
2.1.2.2.1.2.1.2 CAR-T Example Targeting CD20
2.1.2.2.1.2.1.2.1 MustangBio / MB-106
2.1.2.2.1.2.1.3 CAR-T Examples Targeting CD38 molecule on cancer cells
2.1.2.2.1.2.1.3.1 Celgene Bristol Myers Squibb Company. Abecma (idecabtogene vicleucel). FDA Approved.
2.1.2.2.1.2.1.4 CAR-T Examples Targeting CD19 molecule on cancer cells
2.1.2.2.1.2.1.4.1 Kite a Gilead Company. Yescarta. FDA Approved.
2.1.2.2.1.2.1.4.2 Kite a Gilead Company. Tecartus. FDA Approved.
2.1.2.2.1.2.1.4.3 Novartis. Kymriah. FDA Approved.
2.1.2.2.1.2.2 No. Solid tumors. MustangBio / MB-103 (targets HER2)
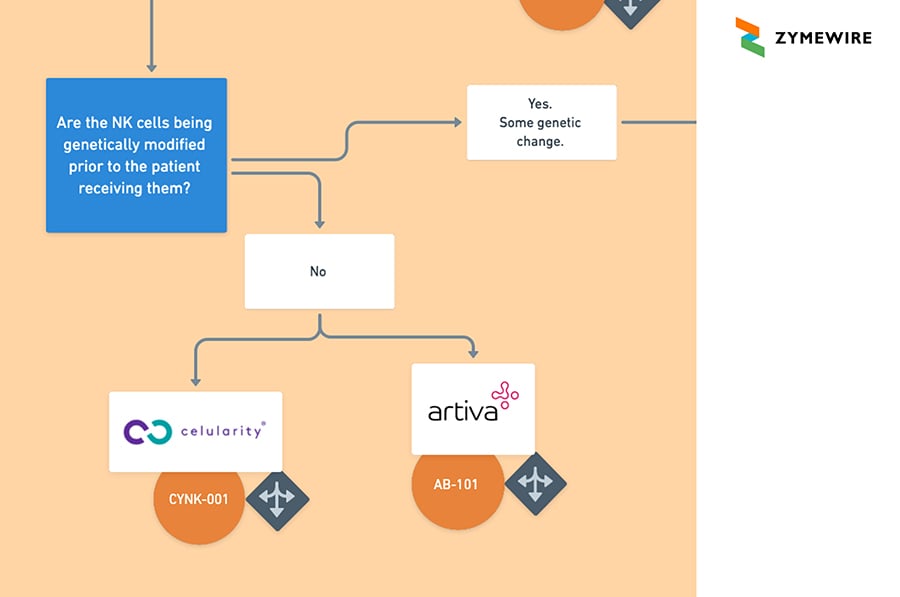
2.1.3. NK Cells (Natural Killer Cells). Are the NK cells being genetically modified prior to the patient receiving them?
2.1.3.1 No. Celularity / CYNK-001. Artiva / AB-101.
2.1.3.2 Yes. Some Genetic Change. Are the NK cells being programmed to express a Chimeric Antigen Receptor?
2.1.3.2.1 Yes. “CAR NK”. Artiva / AB-201 [HER2]. Nkarta Therapeutics / NKX019. Cytovia Therapeutics / CYT-503. ONK Therapeutics / ONKT101.
2.1.3.2.2 No. Other genetic manipulations. Shoreline / preclinical programs. Coeptis Pharmaceuticals / CD38-GEAR-NK (autologous).
2.1.4 Other cell types
2.1.4.1 Immunovative Therapies Ltd. / AlloStim
2.1.4.2 Acepodia / “ACE1831” (y/8 T-cells)
2.1.4.3 Geneta Science / Temeferon (HP stem cells)
2.2. No. Are the cells used for some sort of regeneration treatment?
2.2.1 Yes. Targeting regeneration and repair. Where are the cells from?
2.2.1.1 Other cell types
2.2.1.2 iPSCs. What disease/tissues is the company targeting?
2.2.1.2.1 Liver disease. GC Liver Therapeutics / Hepatocyte product
2.2.1.2.2 Muscular dystrophies. Vita Therapeutics / VTA – 110
2.2.1.2.3 Cardio. Spark Therapeutics / Preclinical candidates
2.2.1.2 Cardiac fibroblasts
2.2.1.2.1 Cardio > Ischemic Heart Dis. Metcela / MTC001
2.2.2 No.
2.2.2.1 Example: sickle cell disease. Graphite Bio / GPH101 - (HSCs modified with functioning version of beta globulin)
2.2.2.2 Example: infectious diseases.
2.2.2.2.1 Respiratory infections Allo Vir – ALVR106
2.2.2.2.2 Infectious diseases > HIV – Antion Biosciences – anti-HIV CAR-T
2. Talks about making genetic changes or uses "gene therapy" terminology. Does the genetic alteration get either incorporated into the cell’s DNA or get replicated to future copies of the cell?2.1 No. Fake out? Not technically a gene therapy. More accurately a Nucleic-acid based therapy or Therapeutic Nucleic Acid.
2.1.1 e.g. The Pfizer and Moderna COVID mRNA vaccines.
2.1.2 Dyne Therapeutics – siRNA enters the cell nucleus but does not alter DNA.
2.2 Yes. Okay, a legit gene therapy. Do they talk about adding a fully working copy of a faulty gene or instructions for a brand new protein?
2.2.1 No new genes. Just tweaks to existing. Additional genes are NOT added to the genome or passed on during cell division. “GENE EDITING”. Targeted breaks are created. Are instructions to repair the breaks given?
2.2.1.1 No. Instructions to repair the break are not given. GENE INACTIVATION (aka silencing knockout or knockdown). How is the genetic material or therapeutic reaching the cells?
2.2.1.1.1 Adeni-Associated Virus (AAV)
2.2.1.1.1.1 Heart Disease> PCSK9 gene. Precision Biosciences
2.2.1.1.2 Lipid nanoparticle “LNP”
2.2.1.1.2.1 Heart Disease> PCSK9 gene. Verve Therapeutics / VERVE-101
2.2.1.2 Yes. Instructions ARE given to correct the function of the gene. GENE INSERTION – new genetic material added during the edit. Is the therapy applied to cells while the cells are inside the patient’s body?
2.2.1.2.1 No. Outside the body (“ex vivo”) Technically a Cell-based Gene Therapy.
2.2.1.2.1.1 Sickle cell disease. Graphite Bio / GPH101-(HSCs modified with functioning version of beta globulin)
2.2.1.2.2 Yes. “In vivo”. How is the genetic material or therapeutic reaching the cells?
2.2.1.2.2.1 Adeno-Associated Virus (AAV)
2.2.1.2.2.1.1 Metabolic> MMA. LogicBio Therapeutics / LB-001
2.2.1.2.2.2 Lipid nanoparticle “LNP”
2.2.1.3 GENE CORRECTION – no new material added. How is the therapeutic reaching the cells?
2.2.1.3.1 Adeno-Associated Virus (AAV)
2.2.1.3.1.1 Musculoskeletal> DMD. Precision Biosciences
2.2.1.3.2 Lipid Nanoparticle “LNP” (aka non-viral)
2.2.1.3.2.1 Liver > Glycogen storage dis. Beam Therapeutics / Preclinical program
2.2.1.3.3 Electroporation
2.2.1.3.3.1 Hemat. > Sickle Cell Disease. Beam Therapeutics / BEAM-101
2.2.2 Yes. “GENE ADDITION”. Is the therapy applied to cells while the cells are inside the patient’s body?
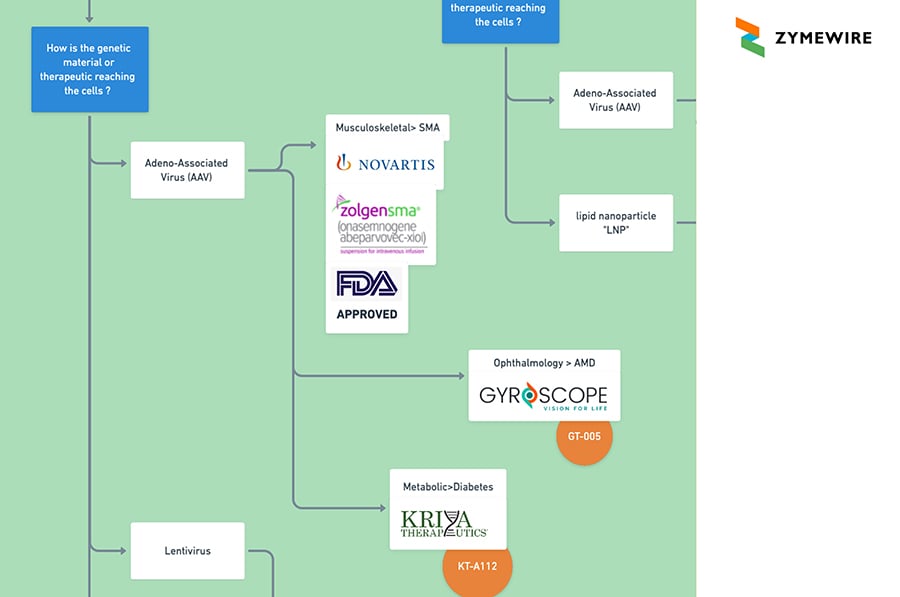
2.2.2.1 Yes. Takes place in Vivo. How is the genetic material or therapeutic reaching the cells?
2.2.2.1.1 Adeno-Associated Virus (AAV)
2.2.2.1.1.1 Musculoskeletal> SM. Novartis. Zolgensma. FDA Approved
2.2.2.1.1.2 Ophthalmology > AMD. Gyroscope Vision for Life / GT-005
2.2.2.1.1.3 Metabolic>Diabetes. Kriya Therapeutics / KT-A112
2.2.2.1.2 Lentivirus
2.2.2.1.2.1 Oncology. Umoja Biopharma / In Vivo CAR-T (new field)
2.2.2.1.3 Liver>PKU
2.2.2.1.3.1 Generation bio / preclinical programs
2.2.2.2 No. It takes place Ex Vivo. Technically a cell-based gene therapy. Is the gene encoding a chimeric antigen T-cell receptor?
2.2.2.2.1 No. Some other gene is being added. Try starting frsh but heading down the Cell Therapy Path.
2.2.2.2.2 Yes. Go to CAR-T.
2.2.2.2.2.1 Infectious Diseases > HIV. Antion Biosciences / anti-HIV CAR-T.
2.2.2.2.2.2 Chimeric Antigen Receptor (CAR) gene(s) added: CAR-T. Are the T-cells the patient receives from her/his own cells?
2.2.2.2.2.2.1 No. From Donors Allogeneic CAR-T (newer fields). Precision Biosciences / PBCARD191 (targets CD19)
2.2.2.2.2.2.2 Yes. Autologous CAR-T “Classic CAR”. Are they targeting a Hematological condition? (blood cancer)
2.2.2.2.2.2.2.1 Yes. Blood cancer. (aka liquid tumors). What is the (antigen) target of the chimeric antigen receptor (CAR)?
2.2.2.2.2.2.2.1.1 CAR-T Examples Targeting BCMA.
2.2.2.2.2.2.2.1.1.1 Legend Biotec / Cartitude-1 Program.
2.2.2.2.2.2.2.1.1.2 Arcellx / CART-ddBCMA
2.2.2.2.2.2.2.1.2 CAR-T Example Targeting CD20
2.2.2.2.2.2.2.1.2.1 MustangBio / MB-106
2.2.2.2.2.2.2.1.3 CAR-T Examples Targeting CD38 molecule on cancer cells
2.2.2.2.2.2.2.1.3.1 Celgene Bristol Myers Squibb Company. Abecma (idecabtogene vicleucel). FDA Approved.
2.2.2.2.2.2.2.1.4 CAR-T Examples Targeting CD19 molecule on cancer cells
2.2.2.2.2.2.2.1.4.1 Kite a Gilead Company. Yescarta. FDA Approved.
2.2.2.2.2.2.2.1.4.2 Kite a Gilead Company. Tecartus. FDA Approved.
2.2.2.2.2.2.2.1.4.3 Novartis. Kymriah. FDA Approved.
2.2.2.2.2.2.2.2 No. Solid tumors. MustangBio / MB-103 (targets HER2)